DRESS syndrome
Drug reaction with eosinophilia and systemic symptoms (DRESS), first described in 1996 by H. Bocquet, is a severe cutaneous reaction associated with eosinophilia and systemic symptoms due to the patient’s susceptibility to certain drugs.1
Epidemiology
The true incidence of DRESS remains unknown because many cases may have been undiagnosed due to its variable clinical presentation and diverse clinical features and laboratory abnormalities.2 The estimated incidence of DRESS syndrome is 9 per million in 1 year, and is known to occur at a frequency of 0.1 to 0.01% after a specific drug exposure.1, 3 The mortality rate of DRESS ranges from 5% to 20%.4
The true incidence of DRESS remains unknown because many cases may have been undiagnosed due to its variable clinical presentation and diverse clinical features and laboratory abnormalities.2 The estimated incidence of DRESS syndrome is 9 per million in 1 year, and is known to occur at a frequency of 0.1 to 0.01% after a specific drug exposure.1, 3 The mortality rate of DRESS ranges from 5% to 20%.4
Etiology
DRESS is known to be caused by a variety of drugs. The common drugs that induce DRESS syndrome are allopurinol (14.7%), carbamazepine (11.9%), vancomycin (7.5%), isoniazid (4.4%), and dapsone (4.2%).3 Antiepileptics (including phenytoin, lamotrigine, and levetiracetam), anti-tuberculosis drugs, antimicrobials (namely, trimethoprim/sulfamethoxazole and other sulfa antimicrobials, beta-lactam antibiotics, glycopeptide antibacterials and fluoroquinolones) can also cause DRESS as well.1, 3 According to recent studies, reactivation of viruses such as human herpesvirus 6 (HHV‐6), human herpesvirus 7 (HHV‐7), Epstein‐Barr virus (EBV), and cytomegalovirus (CMV) might contribute to the immune system dysregulation resulting in DRESS syndrome.2,5
DRESS is known to be caused by a variety of drugs. The common drugs that induce DRESS syndrome are allopurinol (14.7%), carbamazepine (11.9%), vancomycin (7.5%), isoniazid (4.4%), and dapsone (4.2%).3 Antiepileptics (including phenytoin, lamotrigine, and levetiracetam), anti-tuberculosis drugs, antimicrobials (namely, trimethoprim/sulfamethoxazole and other sulfa antimicrobials, beta-lactam antibiotics, glycopeptide antibacterials and fluoroquinolones) can also cause DRESS as well.1, 3 According to recent studies, reactivation of viruses such as human herpesvirus 6 (HHV‐6), human herpesvirus 7 (HHV‐7), Epstein‐Barr virus (EBV), and cytomegalovirus (CMV) might contribute to the immune system dysregulation resulting in DRESS syndrome.2,5
Latency Period
The latency period of DRESS syndrome was longer than that of SJS/TEN.3 Symptoms typically present 2 to 8 weeks after exposure to a culprit medication.1,6 In the recent study led by KoSCAR, the latency periods of allopurinol- and carbamazepine-induced DRESS were observed on 29 days (20-36 days) and 32 days (21-40 days), respectively.3 The RegiSCAR study showed similar latent periods with these drugs: 20 days (17-30 days) for allopurinol and 29 days (20-36 days) for carbamazepine in DRESS.7
The latency period of DRESS syndrome was longer than that of SJS/TEN.3 Symptoms typically present 2 to 8 weeks after exposure to a culprit medication.1,6 In the recent study led by KoSCAR, the latency periods of allopurinol- and carbamazepine-induced DRESS were observed on 29 days (20-36 days) and 32 days (21-40 days), respectively.3 The RegiSCAR study showed similar latent periods with these drugs: 20 days (17-30 days) for allopurinol and 29 days (20-36 days) for carbamazepine in DRESS.7
Risk factors
The risk of developing DRESS is known to be high if a certain genetic predisposition exists. In Koreans, the following associations have been found between the drug and the HLA genotype: allopurinol and HLA-B*58:01, carbamazepine and HLA-A*31:01, antituberculosis drugs and HLA-C*04:01, dapsone and HLA-B*13:01.3 While vancomycin-induced DRESS was associated with HLA-A*32:01 in Europeans, it has not been validated in Koreans.4 Further research is needed on the genetic predisposition that may contribute to the development of DRESS syndrome.
The risk of developing DRESS is known to be high if a certain genetic predisposition exists. In Koreans, the following associations have been found between the drug and the HLA genotype: allopurinol and HLA-B*58:01, carbamazepine and HLA-A*31:01, antituberculosis drugs and HLA-C*04:01, dapsone and HLA-B*13:01.3 While vancomycin-induced DRESS was associated with HLA-A*32:01 in Europeans, it has not been validated in Koreans.4 Further research is needed on the genetic predisposition that may contribute to the development of DRESS syndrome.
Clinical manifestation
It classically presents a morbilliform skin rash that frequently involves 50% of the body surface area, systemic symptoms such as fever of >38°C, lymphadenopathy, and dysfunction of organ such as liver or kidney.1 The cutaneous lesions usually begin as patchy erythematous maculopapular eruptions and become confluent. The lesions are symmetrically distributed on the trunk and extremities. The most characteristic cutaneous lesions during the early phase are periorbital and facial edema. Blisters are occasionally present. Mucosal surfaces along with palms and soles are usually spared, but can occasionally show a few lesions.2 Lymphadenopathy can be found favoring the cervical, axillary, or inguinal regions. Moreover, it should be noted that relapses or flare-ups of these symptoms frequently occur even days to weeks after withdrawal of the original causative drug.2
It classically presents a morbilliform skin rash that frequently involves 50% of the body surface area, systemic symptoms such as fever of >38°C, lymphadenopathy, and dysfunction of organ such as liver or kidney.1 The cutaneous lesions usually begin as patchy erythematous maculopapular eruptions and become confluent. The lesions are symmetrically distributed on the trunk and extremities. The most characteristic cutaneous lesions during the early phase are periorbital and facial edema. Blisters are occasionally present. Mucosal surfaces along with palms and soles are usually spared, but can occasionally show a few lesions.2 Lymphadenopathy can be found favoring the cervical, axillary, or inguinal regions. Moreover, it should be noted that relapses or flare-ups of these symptoms frequently occur even days to weeks after withdrawal of the original causative drug.2
Laboratory findings
Peripheral blood eosinophilia, atypical lymphocytosis and abnormal transaminases level are usual findings.1 In the laboratory tests, hypereosinophilia is observed in 85%, a rapid increase of eosinophil count in an individual’s circulating blood above 1,500/uL. Atypical lymphocytes may also be shown on the peripheral blood smear examination. The elevation in liver enzymes occurs in up to 70% of patients with DRESS at the acute phase. The significant increase in serum IgG titers to HHV-6 and the detection of HHV-6 DNA in leukocytes can be observed in DRESS patients within 2 to 3 weeks after onset.2
Peripheral blood eosinophilia, atypical lymphocytosis and abnormal transaminases level are usual findings.1 In the laboratory tests, hypereosinophilia is observed in 85%, a rapid increase of eosinophil count in an individual’s circulating blood above 1,500/uL. Atypical lymphocytes may also be shown on the peripheral blood smear examination. The elevation in liver enzymes occurs in up to 70% of patients with DRESS at the acute phase. The significant increase in serum IgG titers to HHV-6 and the detection of HHV-6 DNA in leukocytes can be observed in DRESS patients within 2 to 3 weeks after onset.2
Diagnosis
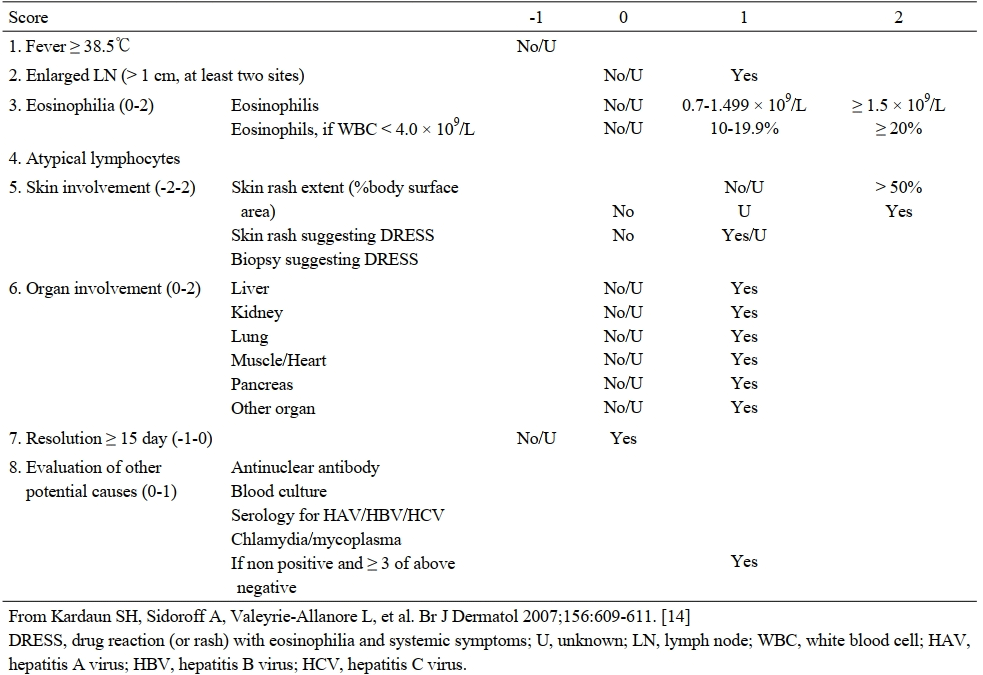
According to the scoring system for classifying DRESS cases (RegiSCAR) reported by Kardaun et al., DRESS syndrome can be diagnosed as follows: fever ≥38.5°C, enlarged lymph nodes, eosinophilia, atypical lymphocytes, skin rash extent >50% body surface area, skin rash suggesting DRESS, biopsy suggesting DRESS, organ involvement (liver, kidney, lung), resolution ≥15 days, viral titers (HBV/HCV) negative (Table 2).8
According to the scoring system for classifying DRESS cases (RegiSCAR) reported by Kardaun et al., DRESS syndrome can be diagnosed as follows: fever ≥38.5°C, enlarged lymph nodes, eosinophilia, atypical lymphocytes, skin rash extent >50% body surface area, skin rash suggesting DRESS, biopsy suggesting DRESS, organ involvement (liver, kidney, lung), resolution ≥15 days, viral titers (HBV/HCV) negative (Table 2).8
Table 2 Scoring system for classifying DRESS of RegiSCAR
In case of diagnostic criteria established by Japanese Research Committee on Severe Cutaneous Adverse Reaction group, HHV-6 reactivation is included for diagnosis of DiHS/DRESS (Table 3).2

In case of diagnostic criteria established by Japanese Research Committee on Severe Cutaneous Adverse Reaction group, HHV-6 reactivation is included for diagnosis of DiHS/DRESS (Table 3).2
Table 3 Diagnostic criteria for DiHS/DRESS established by a Japanese consensus group
|
| The diagnosis is confirmed by the presence of the seven criteria above (typical DiHS/DRESS) or of five of the seven (atypical DiHS/DRESS). †This can be replaced by other organ involvement, such as renal involvement. |
Treatment
To cut off the culprit drug based on the patient’s medical history is of the utmost importance. Systemic corticosteroids have been accepted as the gold standard treatment of DRESS syndrome at the acute phase. The usual dosage is prednisolone, 40-50 mg/day. Systemic corticosteroids, however, need to be tapered over 6-8 weeks to prevent the relapse of various symptoms of this syndrome and thus are administered for 2-3 months. This is because patients with DRESS are at greater risk of subsequently developing the wide spectrum of immune reconstitution inflammatory syndrome (IRIS) ranging from CMV disease to autoimmune disease. IRIS is an increasingly recognized disease concept and is observed with a broad-spectrum of immunosuppressive therapy-related opportunistic infectious disease.2
To cut off the culprit drug based on the patient’s medical history is of the utmost importance. Systemic corticosteroids have been accepted as the gold standard treatment of DRESS syndrome at the acute phase. The usual dosage is prednisolone, 40-50 mg/day. Systemic corticosteroids, however, need to be tapered over 6-8 weeks to prevent the relapse of various symptoms of this syndrome and thus are administered for 2-3 months. This is because patients with DRESS are at greater risk of subsequently developing the wide spectrum of immune reconstitution inflammatory syndrome (IRIS) ranging from CMV disease to autoimmune disease. IRIS is an increasingly recognized disease concept and is observed with a broad-spectrum of immunosuppressive therapy-related opportunistic infectious disease.2
Complications
The prognosis of DRESS, either short-term or long-term, is highly variable and unpredictable. Complications occurring during DRESS include myocarditis, Pneumocystis jirovecii pneumonia, sepsis, and gastrointestinal bleeding, most of which led to significant morbidity and mortality.2 DRESS can also lead dysfunction of the invading organs, typical of liver failure. In case of fulminant hepatic failure, liver transplantation may be required. Skin rashes may devolve into chronic exfoliative dermatitis.3
The prognosis of DRESS, either short-term or long-term, is highly variable and unpredictable. Complications occurring during DRESS include myocarditis, Pneumocystis jirovecii pneumonia, sepsis, and gastrointestinal bleeding, most of which led to significant morbidity and mortality.2 DRESS can also lead dysfunction of the invading organs, typical of liver failure. In case of fulminant hepatic failure, liver transplantation may be required. Skin rashes may devolve into chronic exfoliative dermatitis.3

Reference
- Moran-Marinos C, Alva-Diaz C, De la Cruz Ramirez W, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS) induced by phenytoin re-exposure: case report and systematic review. Acta Clin Belg 2020: 1-9.
- Shiohara T, Mizukawa Y. Drug-induced hypersensitivity syndrome (DiHS)/drug reaction with eosinophilia and systemic symptoms (DRESS): An update in 2019. Allergol Int 2019; 68(3): 301-8.
- Kang DY, Yun J, Lee SY, et al. A Nationwide Study of Severe Cutaneous Adverse Reactions Based on the Multicenter Registry in Korea. J Allergy Clin Immunol Pract 2020.
- Konvinse KC, Trubiano JA, Pavlos R, et al. HLA-A*32:01 is strongly associated with vancomycin-induced drug reaction with eosinophilia and systemic symptoms. J Allergy Clin Immunol 2019; 144(1): 183-92.
- Oberlin KE, Rahnama-Moghadam S, Alomari AK, Haggstrom AN. Drug reaction with eosinophilia and systemic symptoms: Pediatric case series and literature review. Pediatr Dermatol 2019; 36(6): 887-92.
- Ksouda K, Affes H, Mahfoudh N, et al. HLA-A*31:01 and carbamazepine-induced DRESS syndrom in a sample of North African population. Seizure 2017; 53: 42-6.
- Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol 2013; 169(5): 1071-80.
- Herman A, Matthews M, Mairlot M, et al. Drug reaction with eosinophilia and systemic symptoms syndrome in a patient with COVID-19. J Eur Acad Dermatol Venereol 2020; 34(12): e768-e00.

