Stevens-Johnson syndrome/Toxic epidermal necrolysis
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are skin and mucosal abnormalities characterized by rashes and characteristic skin detachment with blisters. SJS was first described in 1922 by A.M. Stevens and F.C. Johnson, and the term TEN was first coined by A. Lyell in 1956.1 SJS, overlap SJS/TEN, and TEN occur along a spectrum of disease. The sub-classification of SJS/TEN is based on the extent of epidermal detachment. If less than 10% of body surface area is affected, it is called SJS, if it is 10 to 30%, it is called overlap SJS/TEN, and if it is more than 30% it is called TEN.
Epidemiology
The global incidence rate associated with TEN is low, estimated between 2 and 13 per million persons yearly.2, 3 In Koreans, the incidences of SJS and TEN are low at 3.96-5.03 people per million in SJS and 0.94-1.45 people per million in TEN, which vary depending on the drug involved.4
SJS is estimated to have a mortality rate of 10% and TEN a mortality rate of 25-35%. Based on data collected in Europe, the estimated mortality of SJS/TEN is 23% at 6 weeks and this increased to 34% at 1 year.5 In the recent study led by the KoSCAR, the overall mortality rate of SJS/TEN was 8.9% in Korean population. While lower than the initial estimation, this mortality rate is still gravely high. Additionally, the vast majority (90%) of deaths occur within two months of the disease, and even if recovered, the morbidity rate of those who suffered from sequalae is high.4
The global incidence rate associated with TEN is low, estimated between 2 and 13 per million persons yearly.2, 3 In Koreans, the incidences of SJS and TEN are low at 3.96-5.03 people per million in SJS and 0.94-1.45 people per million in TEN, which vary depending on the drug involved.4
SJS is estimated to have a mortality rate of 10% and TEN a mortality rate of 25-35%. Based on data collected in Europe, the estimated mortality of SJS/TEN is 23% at 6 weeks and this increased to 34% at 1 year.5 In the recent study led by the KoSCAR, the overall mortality rate of SJS/TEN was 8.9% in Korean population. While lower than the initial estimation, this mortality rate is still gravely high. Additionally, the vast majority (90%) of deaths occur within two months of the disease, and even if recovered, the morbidity rate of those who suffered from sequalae is high.4
Etiology
Medications are the most common cause of SJS/TEN. In a large retrospective cohort study, 89.7% of cases were attributable to a specific medication, whereas 3.4% were secondary to an infectious trigger, and 6.9% were classified as due to unknown cause.3, 6 Common culprit drugs include allopurinol (13.3%), carbamazepine (7.3%), amoxicillin (4.9%), lamotrigine (3.9%), and cefaclor (3.4%).4 Anti-epileptics (including valproic acid and phenytoin), anti-tuberculosis drugs, anti-microbials (namely, trimethoprim/sulfamethoxazole and other sulfa antimicrobials, beta-lactam antibiotics, and fluoroquinolones), anti-inflammatory agents (particularly oxicam), and carbonic anhydrase inhibitors can also cause SJS/TEN as well.3
Medications are the most common cause of SJS/TEN. In a large retrospective cohort study, 89.7% of cases were attributable to a specific medication, whereas 3.4% were secondary to an infectious trigger, and 6.9% were classified as due to unknown cause.3, 6 Common culprit drugs include allopurinol (13.3%), carbamazepine (7.3%), amoxicillin (4.9%), lamotrigine (3.9%), and cefaclor (3.4%).4 Anti-epileptics (including valproic acid and phenytoin), anti-tuberculosis drugs, anti-microbials (namely, trimethoprim/sulfamethoxazole and other sulfa antimicrobials, beta-lactam antibiotics, and fluoroquinolones), anti-inflammatory agents (particularly oxicam), and carbonic anhydrase inhibitors can also cause SJS/TEN as well.3
Latency Period
Typically, culprit drugs have been initiated within the 1 to 3 weeks before the onset of signs and clinical manifestations.4 Development of SJS/TEN is unlikely after the first 8 weeks of treatment with a medication.3
Typically, culprit drugs have been initiated within the 1 to 3 weeks before the onset of signs and clinical manifestations.4 Development of SJS/TEN is unlikely after the first 8 weeks of treatment with a medication.3
Risk factors
SJS/TEN can present in any age group but occurs more frequently in women, HIV-Infected patients, and the elderly. Risk factors include immune dysregulation, such as with HIV infection (100-fold increase), autoimmune disease such as systemic lupus erythematosus (50-fold increase), and active malignancy (particularly hematologic; 30-to-60-fold increase).3
Genetic predisposition can be predicted by certain human leukocyte antigen (HLA) types. HLA-B*58:01 is associated with SJS/TEN due to allopurinol. HLA-B*15:02 is known as a risk marker of carbamazepine-induced SJS/TEN in Han Chinese. However, HLA-B*15:02 is not effective as a risk marker for predicting carbamazepine-induced SJS/TEN due to its low frequency (0.21-0.32%) in Koreans.7 Specific gene-drug associations are closely influenced by the genetic distribution of each ethnic group. Genetic factors predisposing to SJS/TEN are an area of ongoing research interest. Also, additional factors related to cytochrome P450 polymorphisms and other factors associated with decreased medication clearance likely also play a role in increasing risk.3
SJS/TEN can present in any age group but occurs more frequently in women, HIV-Infected patients, and the elderly. Risk factors include immune dysregulation, such as with HIV infection (100-fold increase), autoimmune disease such as systemic lupus erythematosus (50-fold increase), and active malignancy (particularly hematologic; 30-to-60-fold increase).3
Genetic predisposition can be predicted by certain human leukocyte antigen (HLA) types. HLA-B*58:01 is associated with SJS/TEN due to allopurinol. HLA-B*15:02 is known as a risk marker of carbamazepine-induced SJS/TEN in Han Chinese. However, HLA-B*15:02 is not effective as a risk marker for predicting carbamazepine-induced SJS/TEN due to its low frequency (0.21-0.32%) in Koreans.7 Specific gene-drug associations are closely influenced by the genetic distribution of each ethnic group. Genetic factors predisposing to SJS/TEN are an area of ongoing research interest. Also, additional factors related to cytochrome P450 polymorphisms and other factors associated with decreased medication clearance likely also play a role in increasing risk.3
Clinical manifestation
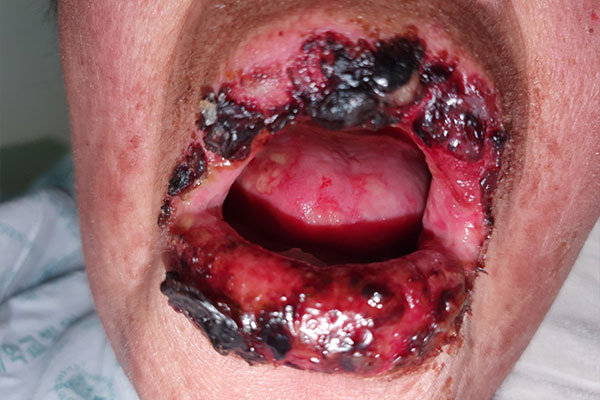
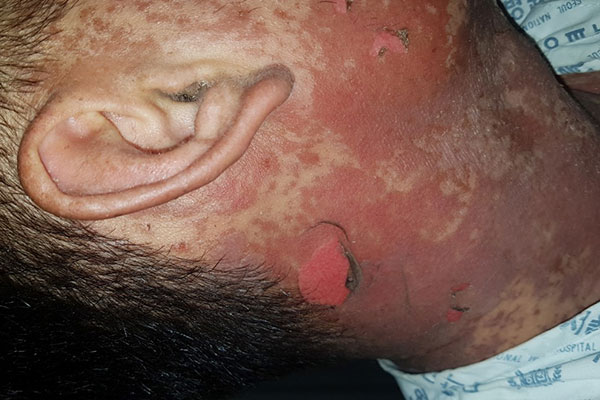
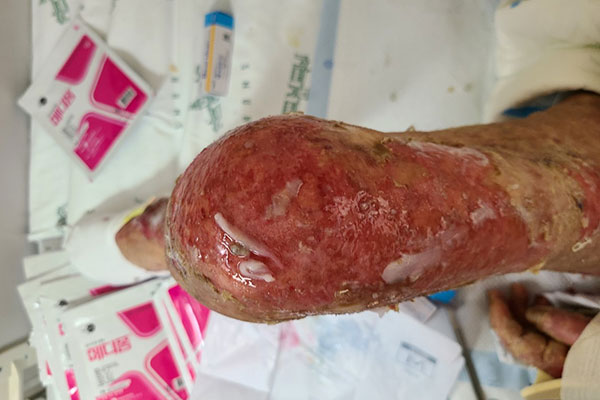
SJS/TEN may manifest as erythematous patches, atypical targetoid lesions, bullae, erosions, and ulcers. The bullae usually show a positive Nikolsky sign: slipping away of the upper layers of the skin from the lower layers when the skin is slightly rubbed. The hallmark feature of SJS/TEN is mucosal involvement (present in 80% of cases), with oral, ocular, genital, or anal mucosa involved. Systemic symptoms, while not uniformly present, may precede skin and mucous membrane findings by 1 to 3 days. Those symptoms may include pain of the skin, eyes, or other mucous membranes, headaches, rhinitis, malaise, sore throat, cough, and myalgias.1
Disease severity and prognosis can be further delineated utilizing the SCORTEN criteria (Table 1). The SCORTEN criteria were introduced in 2000 by Bastuji-Garin et al. and identified seven risk factors that demonstrated excellent agreement between expected and actual mortality rates.1 SCORTEN is an abbreviation of SCORe of TEN, which is a measure of severity of illness for TEN. This score is determined by the number of risk factors that are present. The higher the score is, the greater the mortality rate for the patient. The presence or absence of seven risk factors is used to determine the SCORTEN: (1) age > 40 years, (2) malignancy, (3) total body surface area affected > 10%, (4) heart rate > 120 beats per minute, (5) blood urea nitrogen > 28 mg/dL, (6) serum glucose > 250 mg/dL, (7) serum bicarbonate < 20 mEq/L. The absence of a risk factor is scored as zero; the presence of a risk factor is scored as one. SCORTEN ranges from zero to seven.
SJS/TEN may manifest as erythematous patches, atypical targetoid lesions, bullae, erosions, and ulcers. The bullae usually show a positive Nikolsky sign: slipping away of the upper layers of the skin from the lower layers when the skin is slightly rubbed. The hallmark feature of SJS/TEN is mucosal involvement (present in 80% of cases), with oral, ocular, genital, or anal mucosa involved. Systemic symptoms, while not uniformly present, may precede skin and mucous membrane findings by 1 to 3 days. Those symptoms may include pain of the skin, eyes, or other mucous membranes, headaches, rhinitis, malaise, sore throat, cough, and myalgias.1
Disease severity and prognosis can be further delineated utilizing the SCORTEN criteria (Table 1). The SCORTEN criteria were introduced in 2000 by Bastuji-Garin et al. and identified seven risk factors that demonstrated excellent agreement between expected and actual mortality rates.1 SCORTEN is an abbreviation of SCORe of TEN, which is a measure of severity of illness for TEN. This score is determined by the number of risk factors that are present. The higher the score is, the greater the mortality rate for the patient. The presence or absence of seven risk factors is used to determine the SCORTEN: (1) age > 40 years, (2) malignancy, (3) total body surface area affected > 10%, (4) heart rate > 120 beats per minute, (5) blood urea nitrogen > 28 mg/dL, (6) serum glucose > 250 mg/dL, (7) serum bicarbonate < 20 mEq/L. The absence of a risk factor is scored as zero; the presence of a risk factor is scored as one. SCORTEN ranges from zero to seven.
Table 1 SCORTEN scale
| Risk factors present | Mortality rate (%) |
|---|---|
| 0 - 1 2 3 4 ≥ 5 | 3 12 35 58 90 |
Diagnosis
When SJS/TEN is clinically likely, appropriate therapy should be instituted without delay, even if histologic confirmation is pending; however, a skin biopsy should always be performed for completeness. The characteristic pathologic finding is full-thickness epidermal necrosis. Potential SJS/TEN mimics that should be considered in the differential include severe acute cutaneous lupus, staphylococcal scalded skin syndrome, autoimmune blistering diseases, and grade IV acute graft-versus-host disease.3
When SJS/TEN is clinically likely, appropriate therapy should be instituted without delay, even if histologic confirmation is pending; however, a skin biopsy should always be performed for completeness. The characteristic pathologic finding is full-thickness epidermal necrosis. Potential SJS/TEN mimics that should be considered in the differential include severe acute cutaneous lupus, staphylococcal scalded skin syndrome, autoimmune blistering diseases, and grade IV acute graft-versus-host disease.3
Treatment
Identification and early withdrawal of the offending medication(s) is the most important action in caring for a patient with SJS/TEN. Supportive care in an intensive care unit or burn treatment center is recommended. Fluid replacement with electrolyte solution and albumin solution is required. Appropriate nutritional support is also needed. If an infection is suspected, a broad-spectrum antibiotic treatment is recommended.
Ophthalmologic consultation is essential. Preventive care, including lubrication, topical antibiotics, topical corticosteroids, and lysis of adhesions is effective in reducing ocular sequelae. In patients with genitourinary involvement, urologic consultation is recommended.
There are several adjunctive therapeutic options for SJS/TEN, but their effectiveness is, thus far, underwhelming. TNFα inhibitors and cyclosporine A are promising potential therapies.1
Identification and early withdrawal of the offending medication(s) is the most important action in caring for a patient with SJS/TEN. Supportive care in an intensive care unit or burn treatment center is recommended. Fluid replacement with electrolyte solution and albumin solution is required. Appropriate nutritional support is also needed. If an infection is suspected, a broad-spectrum antibiotic treatment is recommended.
Ophthalmologic consultation is essential. Preventive care, including lubrication, topical antibiotics, topical corticosteroids, and lysis of adhesions is effective in reducing ocular sequelae. In patients with genitourinary involvement, urologic consultation is recommended.
There are several adjunctive therapeutic options for SJS/TEN, but their effectiveness is, thus far, underwhelming. TNFα inhibitors and cyclosporine A are promising potential therapies.1
Complications
Epidermal detachment in SJS/TEN can lead to dehydration, infection (sepsis), ophthalmic problems, respiratory failure, permanent skin damage, and in severe cases, death. Sixty-five percent of SJS/TEN patients suffer from long-term complications such as ocular synechia, corneal damage, vision loss, perineal stricture, bronchiolitis, and hair loss. Patients also report developing post-traumatic stress disorder due to the mental toll the disease takes.4
Epidermal detachment in SJS/TEN can lead to dehydration, infection (sepsis), ophthalmic problems, respiratory failure, permanent skin damage, and in severe cases, death. Sixty-five percent of SJS/TEN patients suffer from long-term complications such as ocular synechia, corneal damage, vision loss, perineal stricture, bronchiolitis, and hair loss. Patients also report developing post-traumatic stress disorder due to the mental toll the disease takes.4
Reference
- Schneider JA, Cohen PR. Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Concise Review with a Comprehensive Summary of Therapeutic Interventions Emphasizing Supportive Measures. Adv Ther 2017; 34(6): 1235-44.
- Fakoya AOJ, Omenyi P, Anthony P, et al. Stevens - Johnson Syndrome and Toxic Epidermal Necrolysis; Extensive Review of Reports of Drug-Induced Etiologies, and Possible Therapeutic Modalities. Open Access Maced J Med Sci 2018; 6(4): 730-8.
- Noe MH, Micheletti RG. Diagnosis and management of Stevens-Johnson syndrome/toxic epidermal necrolysis. Clin Dermatol 2020; 38(6): 607-12.
- Kang DY, Yun J, Lee SY, et al. A Nationwide Study of Severe Cutaneous Adverse Reactions Based on the Multicenter Registry in Korea. J Allergy Clin Immunol Pract 2020.
- Lee HY, Chung WH. Toxic epidermal necrolysis: the year in review. Curr Opin Allergy Clin Immunol 2013; 13(4): 330-6.
- Micheletti RG, Chiesa-Fuxench Z, Noe MH, et al. Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis: A Multicenter Retrospective Study of 377 Adult Patients from the United States. J Invest Dermatol 2018; 138(11): 2315-21.
- Kim SH, Lee KW, Song WJ, et al. Carbamazepine-induced severe cutaneous adverse reactions and HLA genotypes in Koreans. Epilepsy Res 2011; 97(1-2): 190-7.

